【转载】贵州大学池永贵/伍星星/山东大学胡钧员ACS Catal.:有机催化前手性次磷酸的不对称甲基化构建五价磷手性中心
2025-12-10 浏览次数: 38
贵州大学池永贵/伍星星/山东大学胡钧员ACS Catal.:有机催化
前手性次磷酸的不对称甲基化构建五价磷手性中心
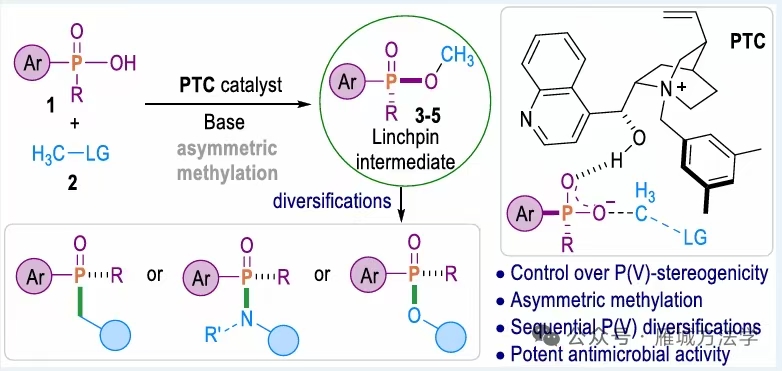
图1. TOC (图片来源于ACS Catal.)
研究背景:
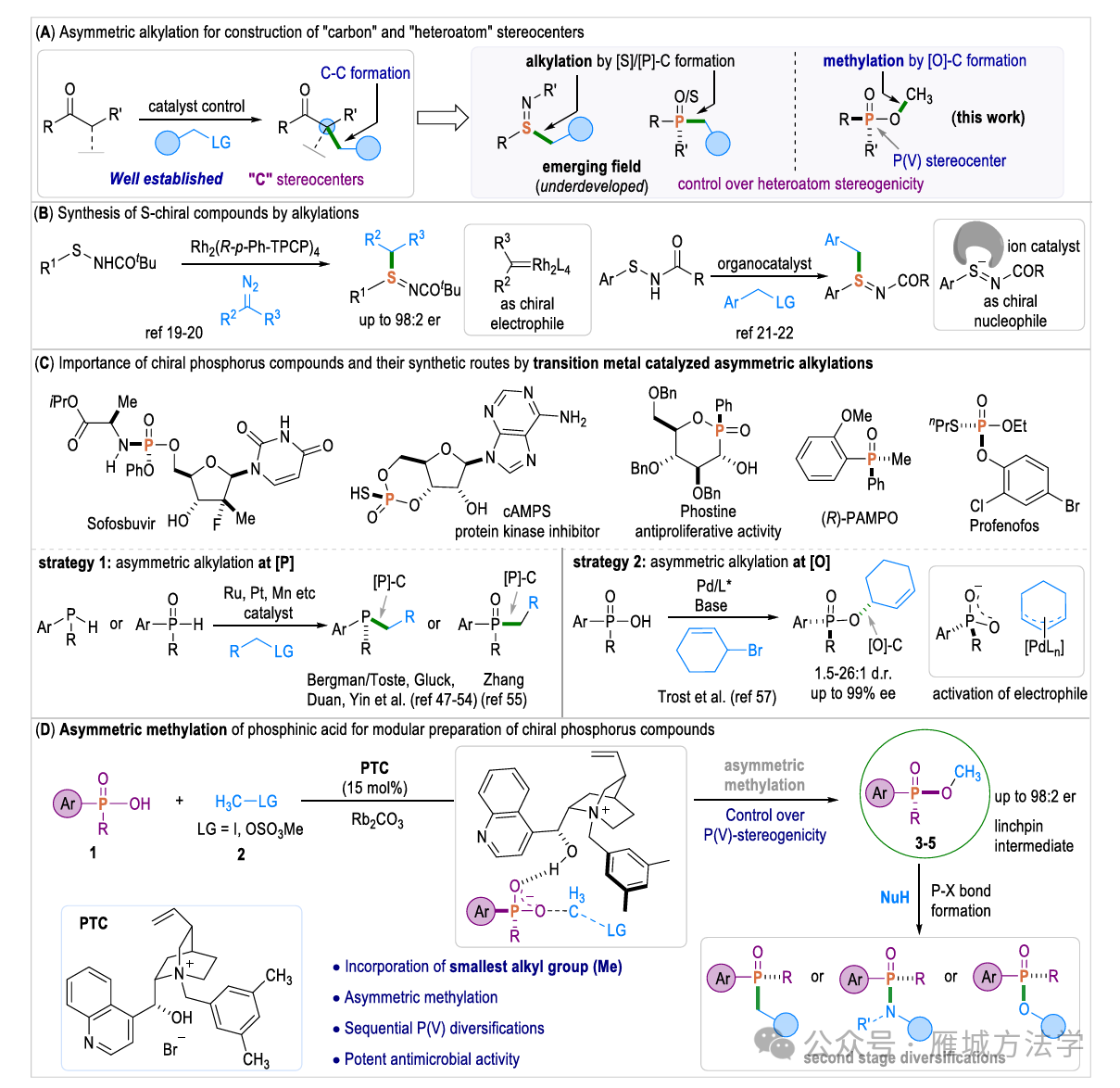
研究内容:
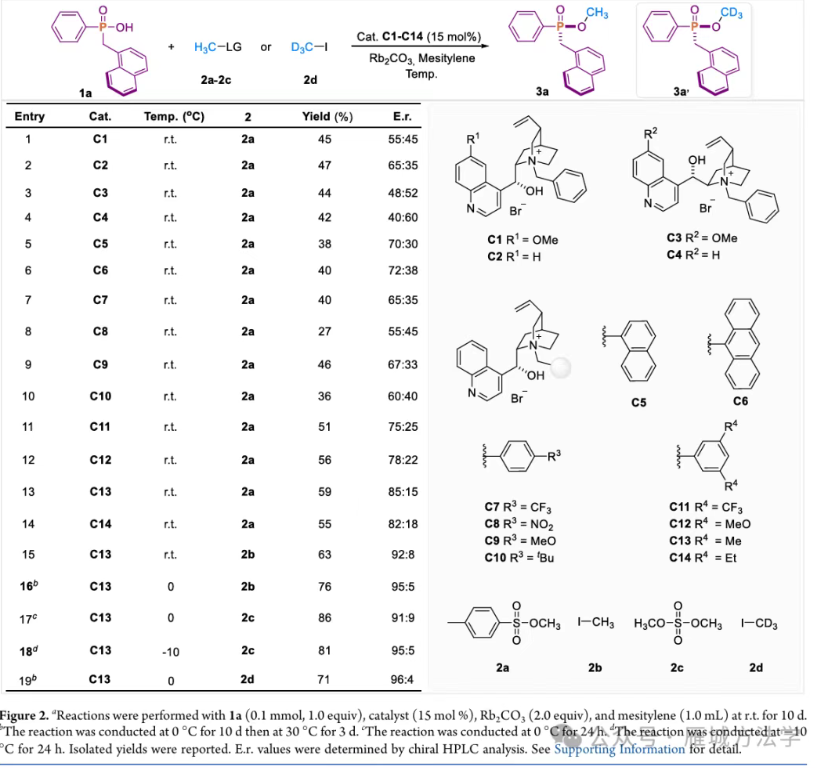
图3. 条件优化 (图片来源于ACS Catal.)
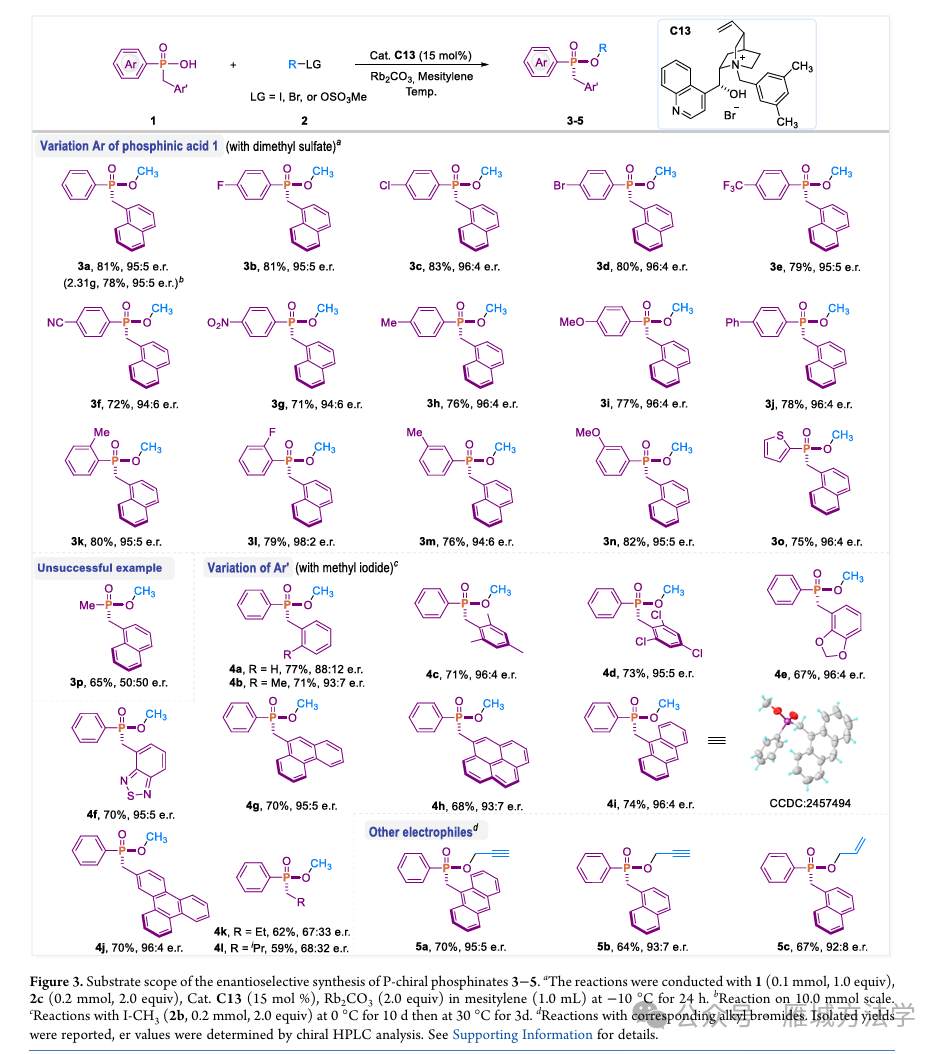
图4. 底物拓展 (图片来源于ACS Catal.)
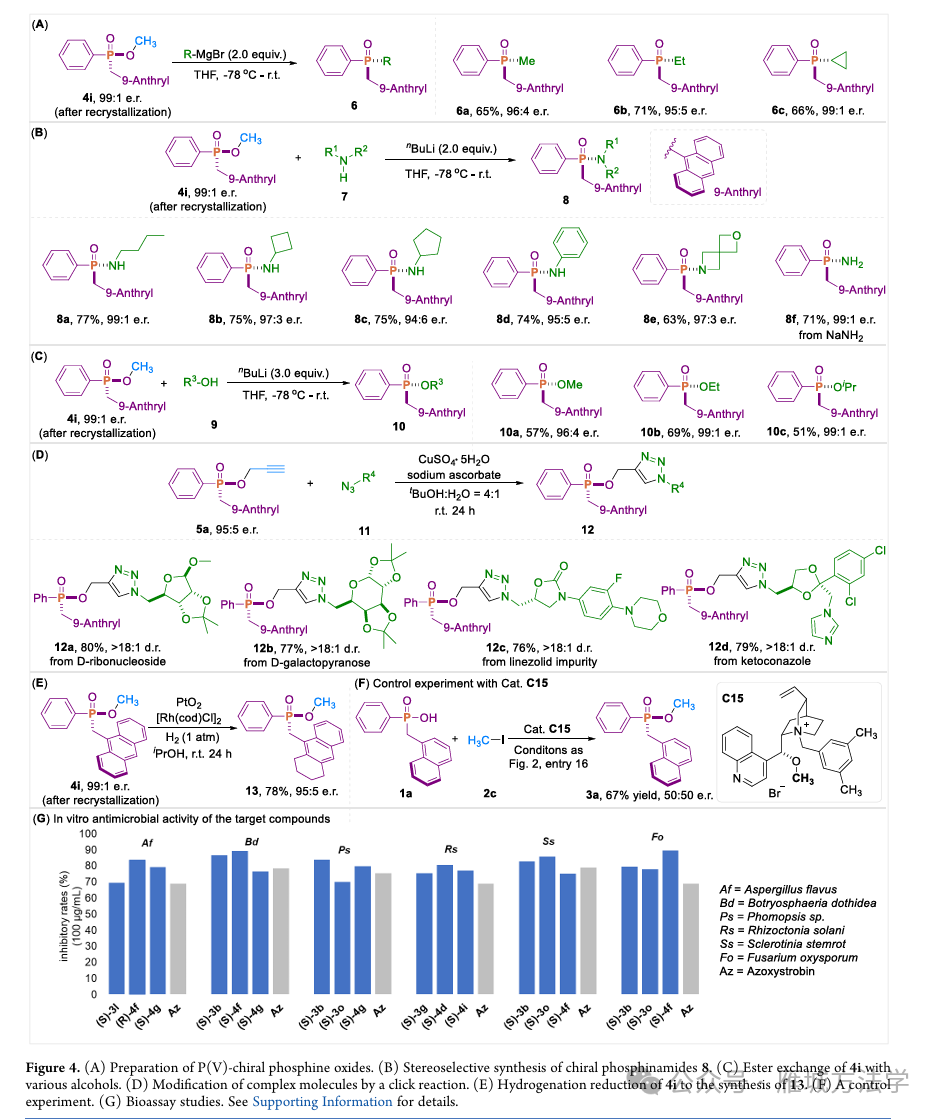
图5. 制备手性膦氧化合物、立体选择性合成手性磷酰胺、控制实验等及抗菌活性测试
(图片来源于ACS Catal.)
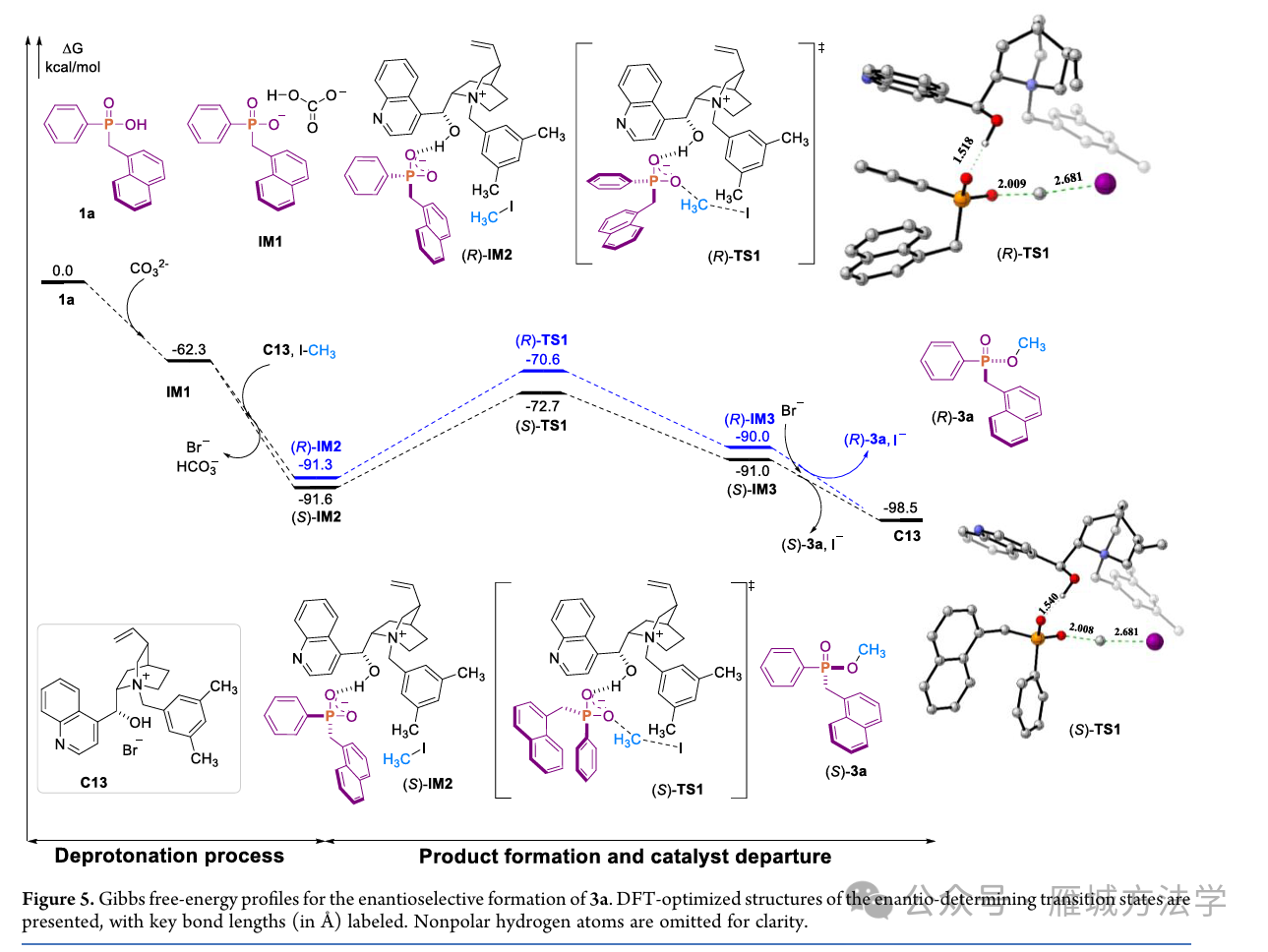
In summary, we have developed an organocatalytic asymmetric alkylation strategy for the synthesis of P(V)-stereogenic phosphorus compounds via stereoselective O−C bond formation. Utilizing a cinchonidine-derived phase-transfer catalyst, an ion pair is formed between the chiral catalyst and a prochiral phosphinic acid anion, generating a chiral environment that enables high stereocontrol over methylation with simple methylating agents. This mild and efficient protocol affords a broad array of chiral phosphorus compounds with high enantioselectivity, thereby expanding the synthetic toolbox for P(V)-stereogenic functional molecules. Moreover, the resulting chiral phosphinates serve as versatile linchpin intermediates, undergoing stereospecific nucleophilic substitutions to furnish diverse phosphorus-containing scaffolds, including tertiary phosphine oxides, phosphinamides, and phosphinates. This method not only provides new access to structurally diverse P-chiral compounds but also offers promising candidates for applications in the discovery of agrochemical lead compounds and other biologically relevant fields.
来源于:南华大学