贵州大学伍星星课题组Sci. Adv.:氯代砜亚胺的不对称去氧反应构建含S(IV)手性中心的亚磺酰亚胺酯衍生物
2025-08-19 浏览次数: 10
摘要:
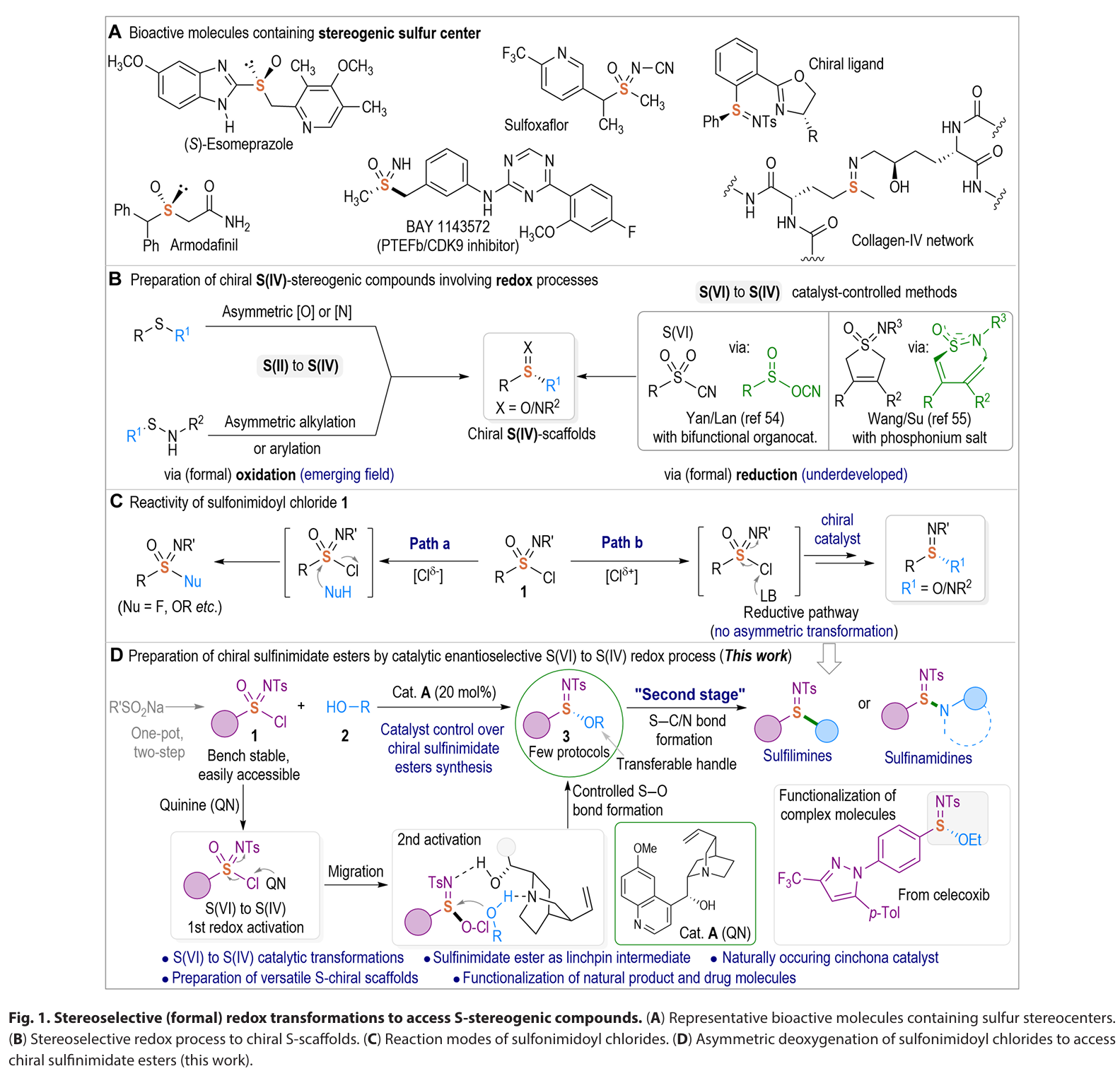
Sulfur plays a pivotal role across diverse fields owing to its distinctive chemical and biological properties, thereby propelling the development of stereoselective synthesis of enantioenriched S(IV)- stereogenic scaffolds. While oxidative strategies for generating S- chiral frameworks have witnessed emerging development, the catalytic re duction of S(VI) species to furnish enantioenriched S(IV) scaffolds remains largely overlooked. Herein, we present an enantioselective catalytic method to access S(IV)-stereogenic sulfinimidate ester scaffolds through a distinct catalytic asymmetric formal reductive transformation of S(VI)-sulfonimidoyl chlorides. Using a naturally occur ring, cost- effective quinine organocatalyst, we achieved high enantioselectivity in chiral sulfinimidate ester formation via asymmetric S─O bond formation. The catalyst plays dual critical roles, facilitating both the reduction of S(VI) substrates and the catalytic stereoselective formation of S─O bonds. This approach offers a rapid and efficient pathway to sulfinimidate esters, which serves as versatile intermediates for subsequent modular access to diverse S- chiral sulfilimine and sulfinamidine scaffolds, with promising applications in agrochemical and pharmaceutical discovery.
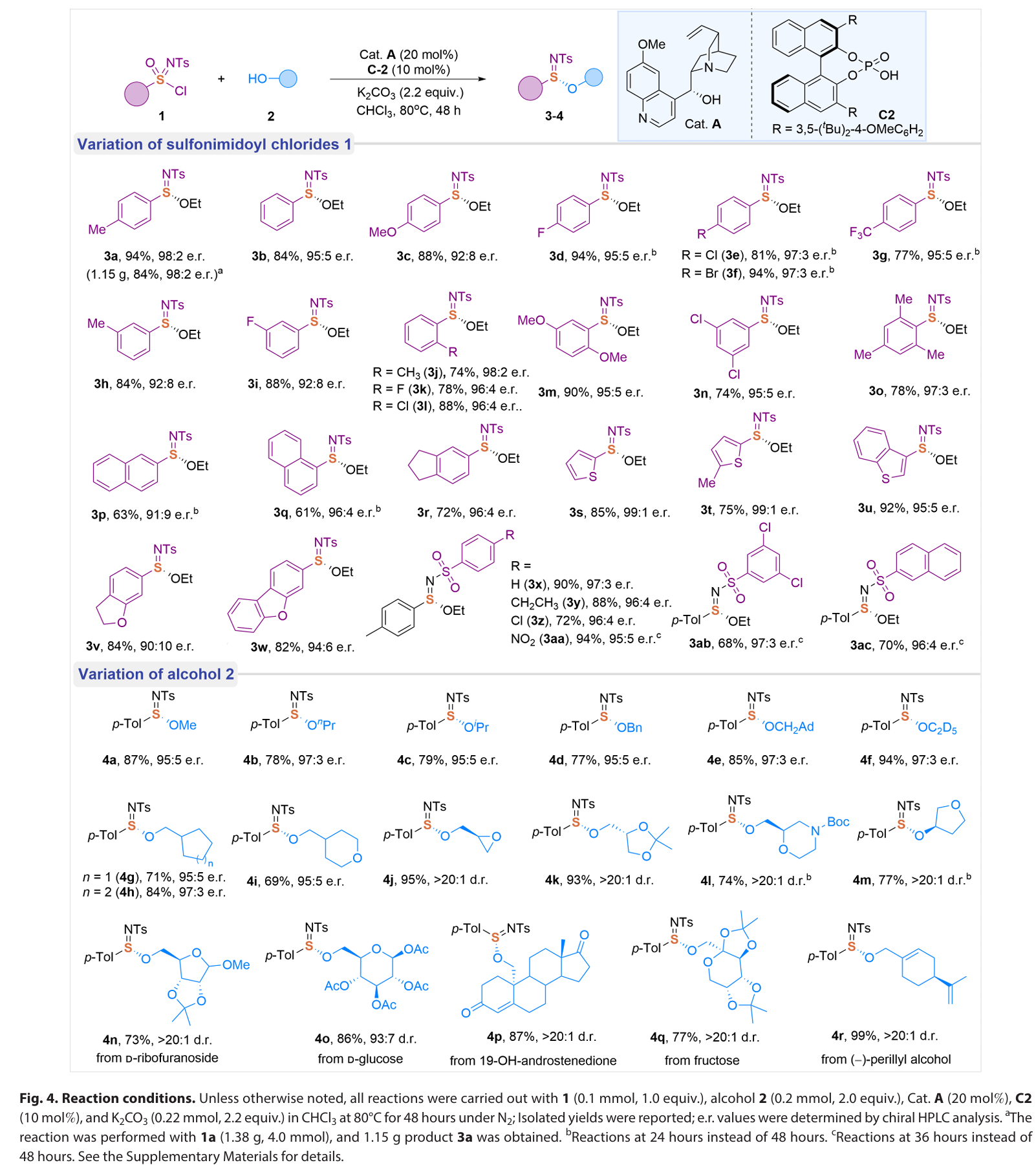
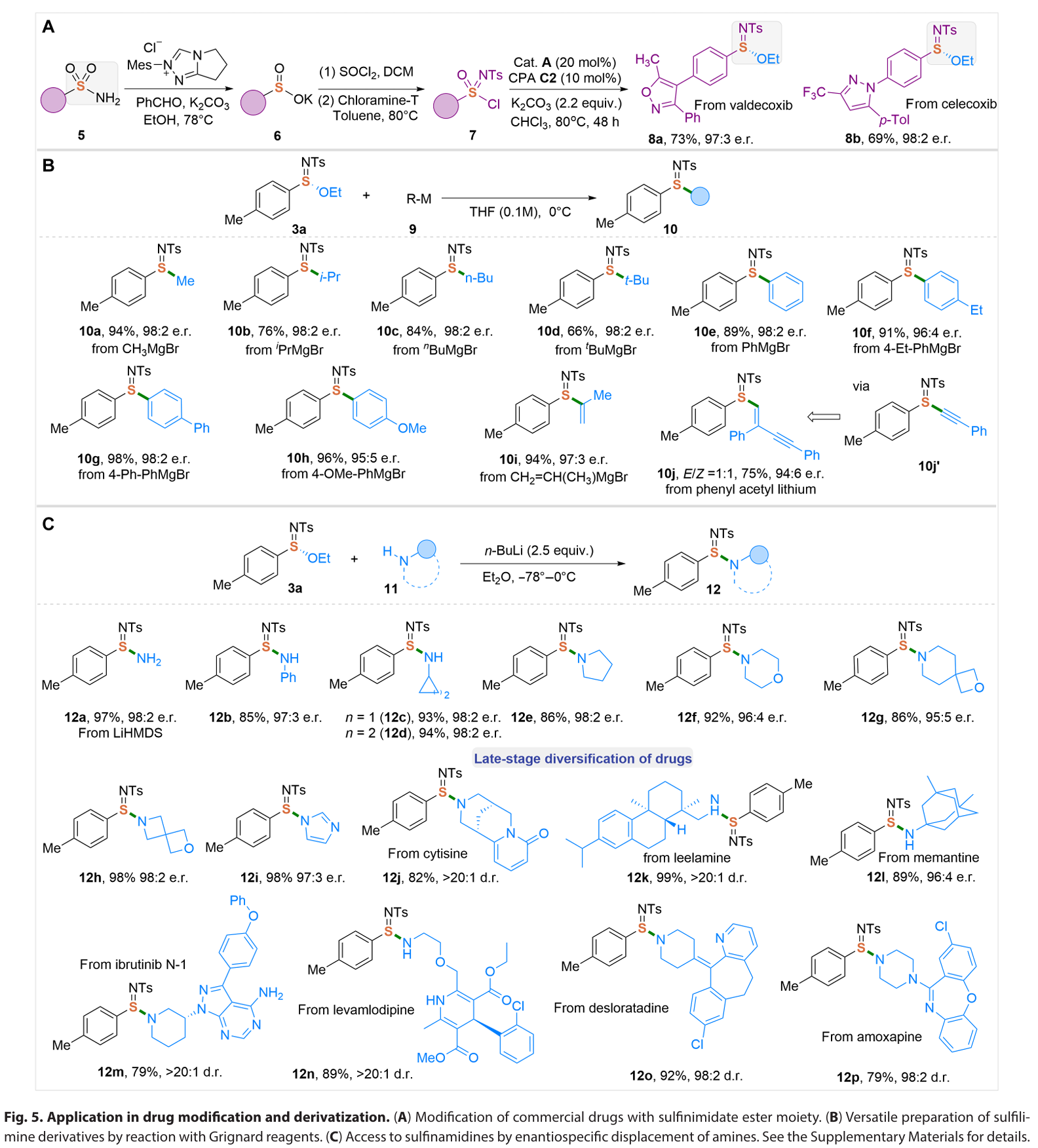
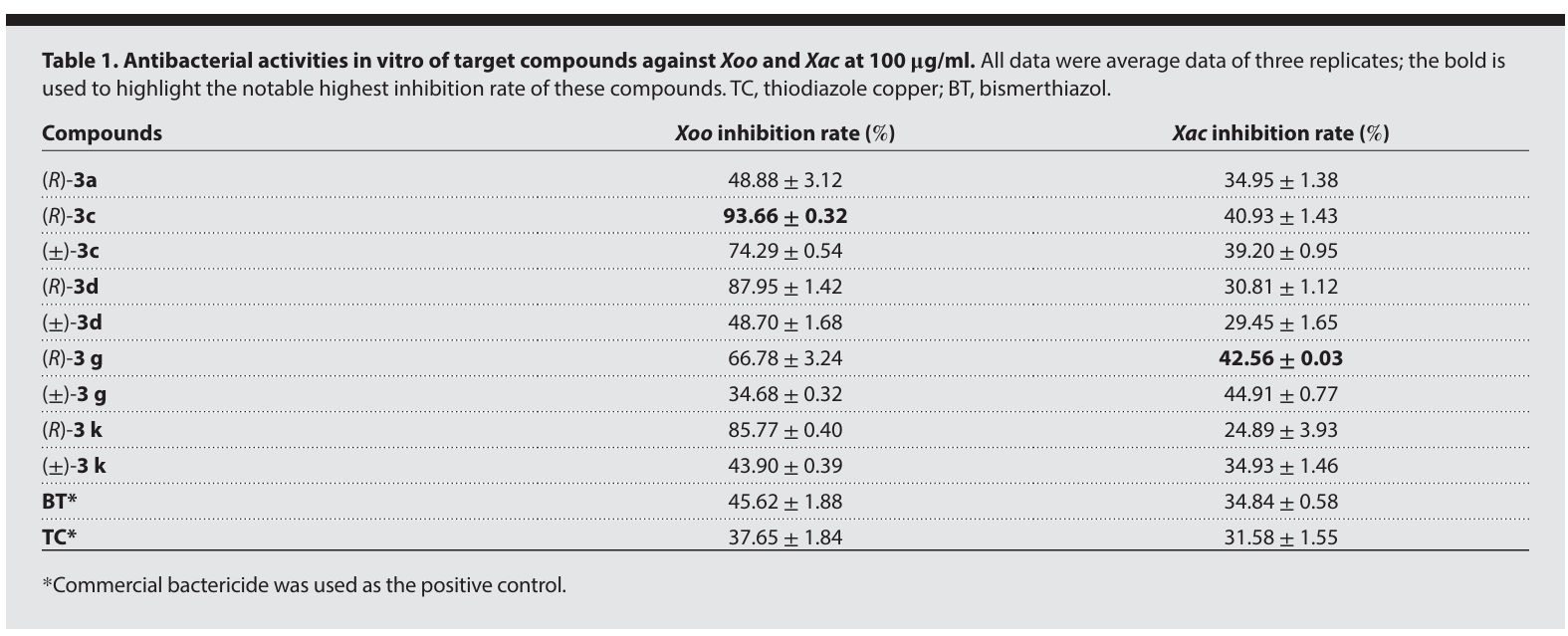
总结:
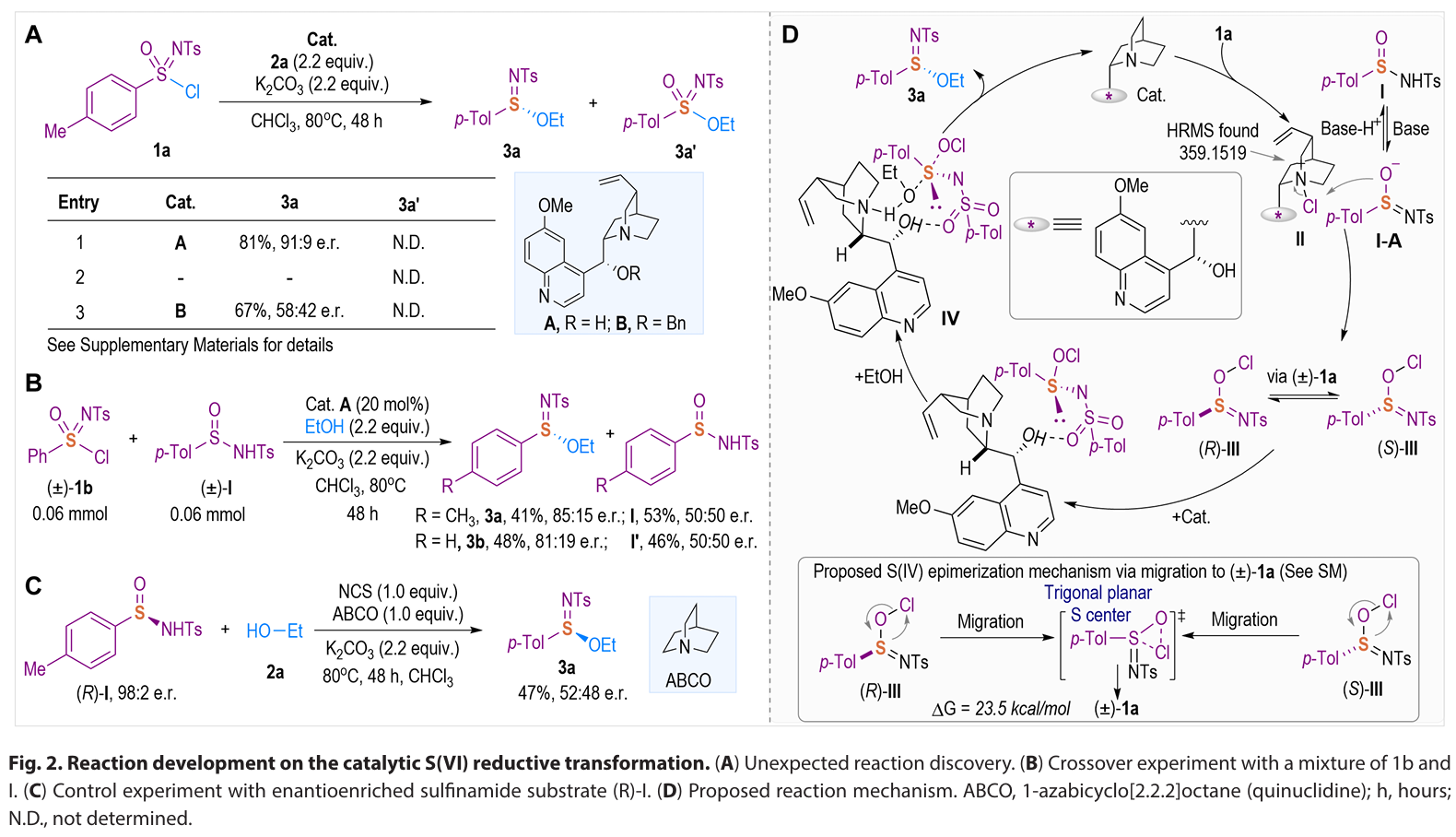
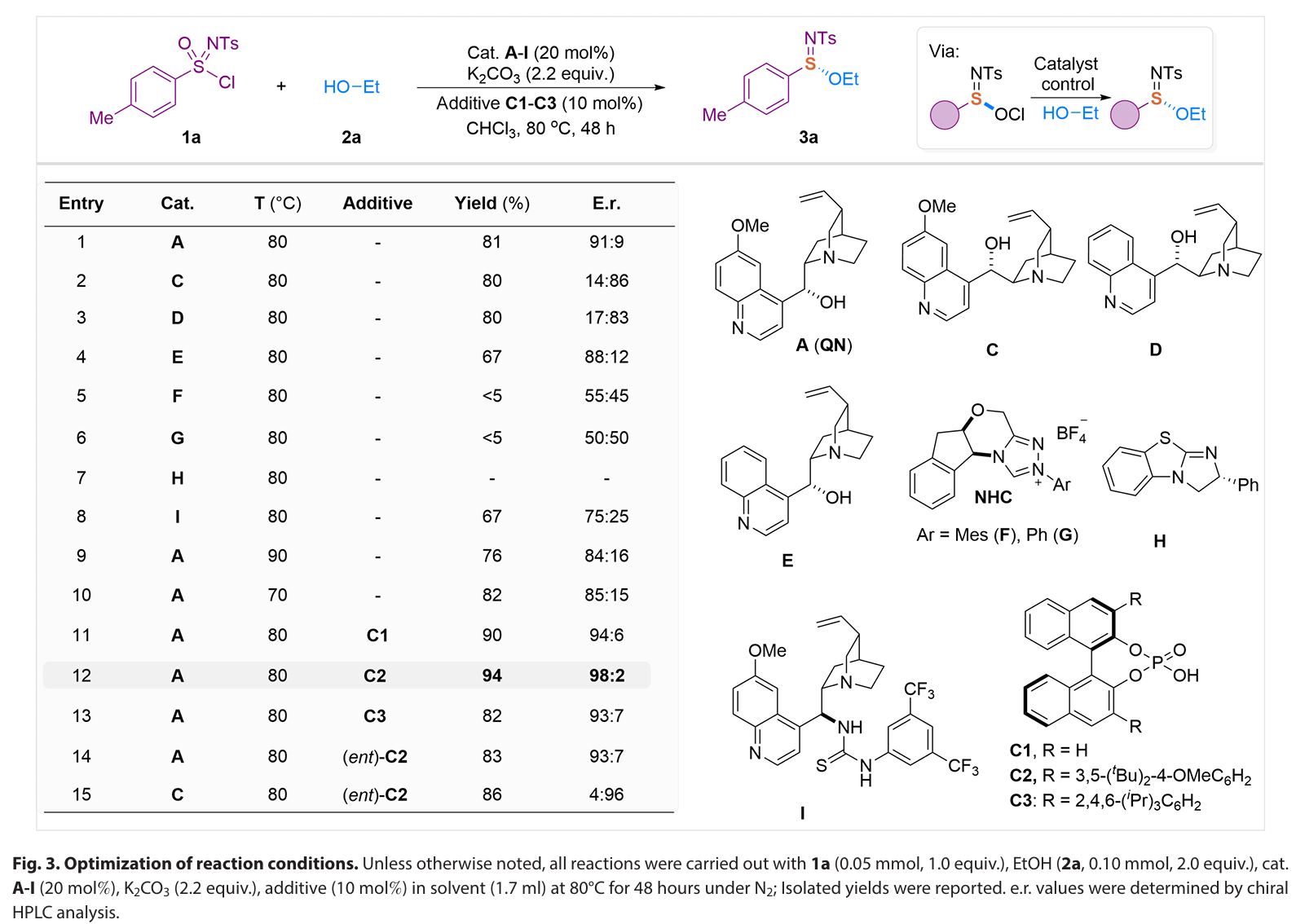
In summary, we have developed an enantioselective catalytic approach for the efficient synthesis of S(IV)- stereogenic sulfinimidate ester scaffolds through a unique catalytic asymmetric formal reductive transformation of S(VI)-sulfonimidoyl chlorides. Governed by a naturally occurring, cost- effective quinine organocatalyst, this method delivers a broad range of chiral sulfinimidate esters with high optical purity through catalyst- controlled asymmetric S─O bond formation. Mechanistically, the quinine catalyst serves dual critical functions: facilitating the reduction of S(VI) to S(IV) through the formation of a covalent catalyst-Cl+ adduct and catalyzing the stereoselective formation of S─O bonds. Furthermore, the sulfinimidates produced by our method represent a linchpin intermediate that allows for sequential stereospecific displacement manipulations for the divergent synthesis of various S- stereogenic scaffolds, such as sulfilimines and sulfinamidines, via S─C/N couplings with an array of natural products and biologically relevant molecules. Ongoing research in our laboratory focuses on advancing the catalytic asymmetric (formal) reduction strategies to address the challenges in preparing sulfur stereogenic compounds, as well as developing S- chiral sulfinimidates for applications in chiral catalyst design and antibacterial agent discovery.
文章信息:
Minghong Liao, Shiqing Huang, Qiang Xiong, Zhongfu Luo, Sha Zhao, Taiyou Xu, Xingxing Wu*. Asymmetric catalytic reductive transformation of S(VI) to S(IV) for modular access to S(IV)- stereogenic sulfilimine derivatives. Sci. Adv. 2025, 11, eadx2509.